
Brief Description
Breaking infection chains in the clinical environment is one of the biggest challenges in the healthcare system. At the same time, however, digitization is also being driven forward here and, for example, patient records are being maintained digitally. Increasingly, data entry and processing is done via smartphone or tablet. These devices often move from hand to hand, easily enabling pathogen transmission. Chemical disinfection of the sensitive devices is not possible due to the aggressiveness of the disinfectants. The aim of the project was to develop a functional model of a UVC-LED-based, safe and above all validatable disinfection of smartphones, tablets and other smaller devices.
Project goals
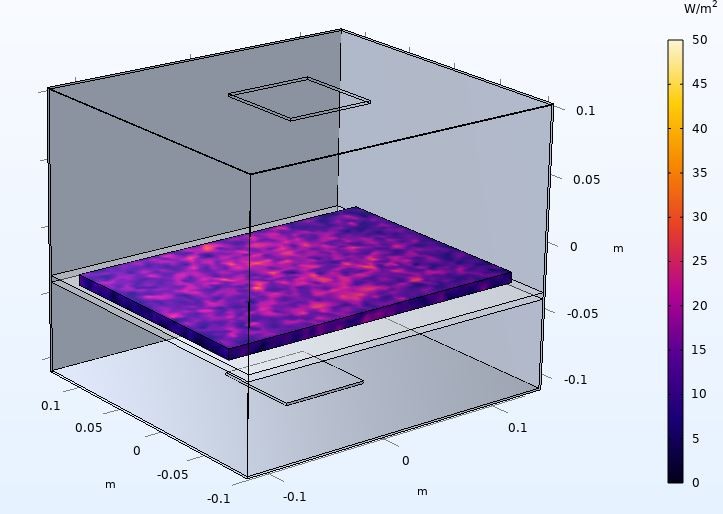
In contrast to smartphone disinfection devices available on the market, the focus of the project was on the validability of the disinfection. The irradiation power should be sufficiently high to achieve an irradiation dose of at least 800 J/m² within one minute and thus an inactivation by five log levels for all relevant hospital pathogens. Furthermore, shadowing should be prevented, irradiation should be uniform, and a clear assignment between disinfection material and disinfection process with logging should be established.
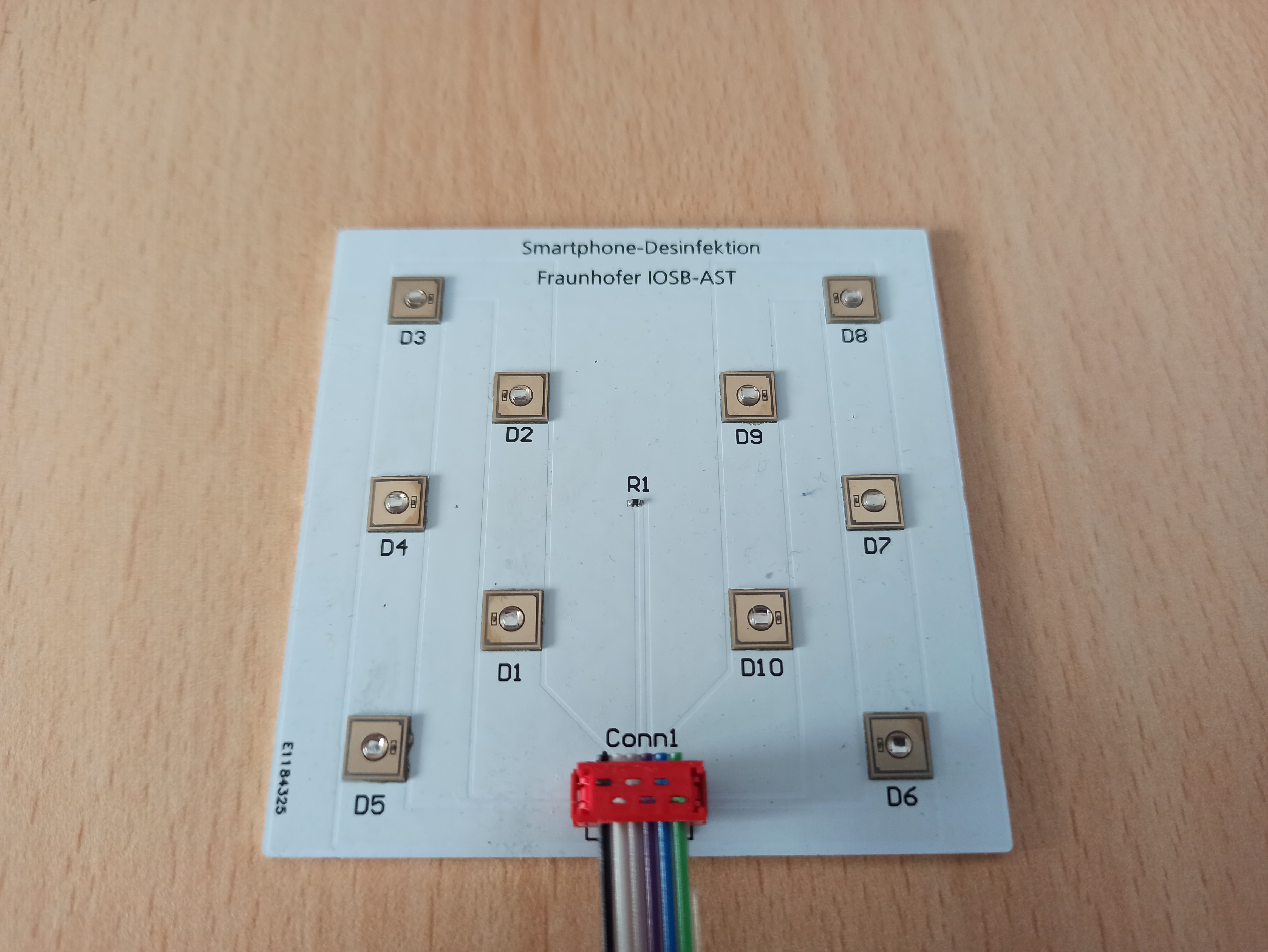
To verify the irradiation, an NFC scanner and several photosensors are installed to identify the device and measure the irradiation dose. The functional sample has a WiFi interface and can be integrated into a database infrastructure via MQTT. The marketing of the device is planned via the industrial partner PURION GmbH. In the process, larger devices are also to be launched on the market.
Project result
Together with Boehringer-Ingelheim and the LS laboratory in Bad Bocklet-Großenbrach, the device was subjected to extensive testing in which the microorganisms Staphylococcus aureus. Pseudomonas aeruginosa, Candida albicans, Aspergillus brasiliensis niger, Bacillus subtilis and Paenibacillus glucanolyticus were irradiated on various surface materials in several test series and the disinfection performance of the device was investigated.
It was demonstrated that bacteria in particular can be validated to be inactivated by up to 7 log levels within one minute. Yeasts and molds are significantly more resistant and require higher irradiation doses, but these can be adjusted on the device.
 Advanced System Technology branch AST of Fraunhofer IOSB
Advanced System Technology branch AST of Fraunhofer IOSB