
Brief description
The validated reprocessing of medical devices is always a challenge in everyday medical practice. In particular, thermolabile endoscopes or devices with screens such as laryngoscopes cannot be autoclaved, but only manually disinfected. This manual process is error-prone and tedious. In most cases, disinfection gels must be evenly distributed on the surface according to the manufacturer's instructions, allowed to act for a certain time, and then removed again in a further process step UV disinfection with high irradiation doses enables efficient, and rapid, inactivation of microorganisms adhering to the device, thus sterilizing it.
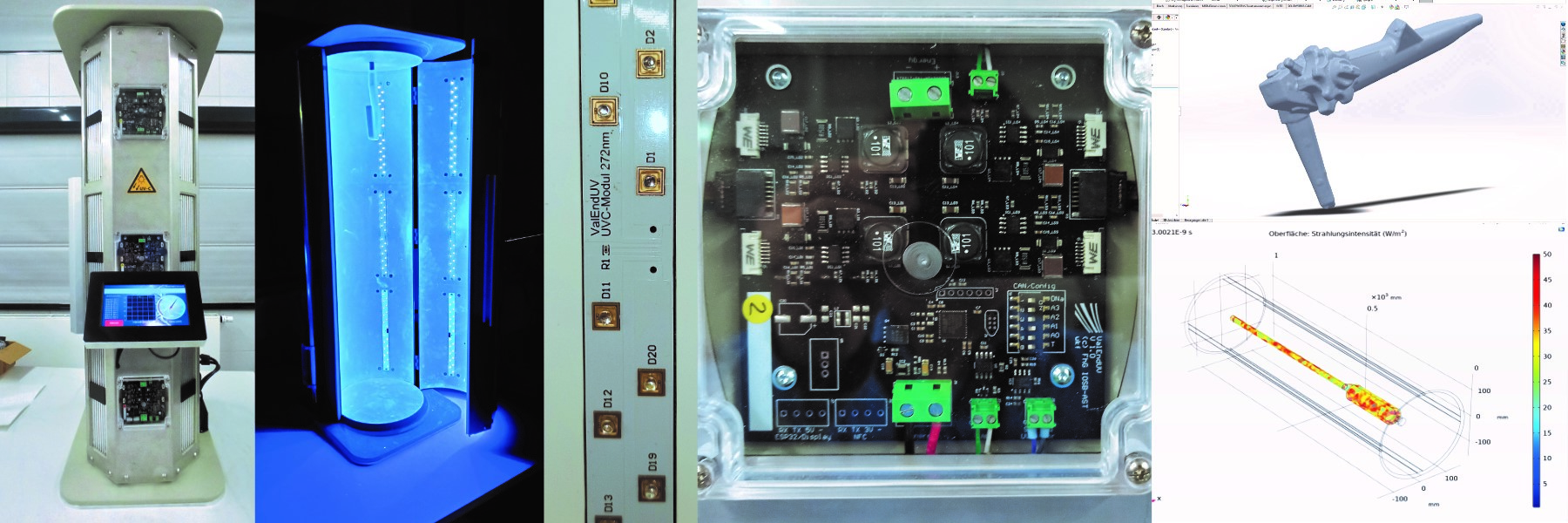
As a leading institute in UV-LED technology, Fraunhofer IOSB-AST has set up a functional model for evaluating the process, which has been microbiologically tested for its functionality and applicability in clinical routine in cooperation with the University Hospital of Jena. By designing the UV radiation sources accordingly, the process time for reprocessing could be reduced to 10 seconds.
Project goals
The aim of the project is to demonstrate that UV radiation generated by modern UVC LEDs is suitable for the safe validated reprocessing of medical devices. In this context, the focus was also on testing for material compatibility, which, however, proved to be unproblematic, since the irradiation dose required for disinfection is significantly lower than that at which material damage occurs.
Validation is achieved by means of photosensors built into the device, which record the actual irradiance and determine the required dose on the basis of the inactivation curves for various microorganisms known for the emission wave lengths used. By means of a built-in NFC scanner, the disinfection process can be assigned to the respective endoscope, which is equipped with an NFC tag. The disinfection processes are logged with time, and dose for traceability.
Project result
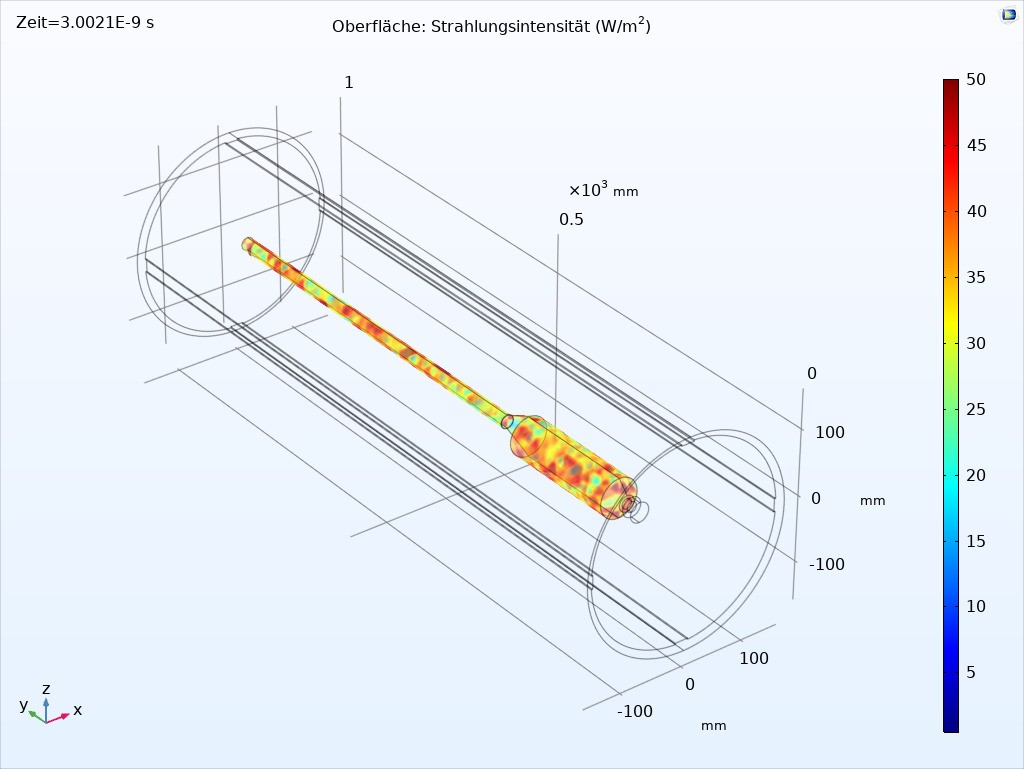
At the beginning of the project, Fraunhofer IOSB-AST developed various UVC radiation sources with different power levels and wavelengths. With these UV sources, extensive irradiation experiments with several microorganisms on the typical surfaces of an endoscope were carried out at the University Hospital Jena in order to gain knowledge about the irradiation doses required for inactivation. Based on these results, various radiation simulations with different UV source arrangements were performed. This led to a device design which is able to provide sufficient and safe disinfection in a very short time.
By lining the interior with optical scattering PTFE, it was also possible to realize homogeneous radiation from all directions, which can also radiate into critical gaps on the handle of the endoscope. The device is designed as a vertical cylinder into which the endoscope is suspended. This ensures that the endoscope is brought into a straight position by gravity and that no support points are required on the endoscope where shadowing could occur.
 Advanced System Technology branch AST of Fraunhofer IOSB
Advanced System Technology branch AST of Fraunhofer IOSB